When it comes to light and color, hydrogen is unique. Unlike other gases, it absorbs certain colors of light while allowing others to pass through. This makes hydrogen ideal for use in lasers and other applications where a specific color of light is needed.
The colors that are absorbed by hydrogen gas are red, orange, yellow, and green. Blue and violet light are not absorbed by hydrogen gas, making them the perfect choice for applications where a white light is desired.
Spectrum Demo: Continuous and Emission
When it comes to light, hydrogen gas is pretty much a blank slate. It doesn’t absorb or emit any colors of light on its own. However, when hydrogen gas is exposed to ultraviolet (UV) light, it can absorb some of that UV light and re-emit it as visible light.
This process is called fluorescence.
What Color of Light are Absorbed by Helium Gas
When it comes to light, different colors are absorbed and reflected in different ways. This is why we see a variety of colors when white light shines on an object – the different colors are being absorbed at different rates. But what about when it comes to gases?
What effect do they have on light?
It turns out that each gas has a unique way of interacting with light. For example, helium gas is known for its ability to absorb red light.
This is because the atoms in helium gas are able to absorb the energy from red photons and convert it into heat. As a result, if you shine a red light on helium gas, it will appear dark.
But why does this happen?
It all has to do with the way that electrons interact with photons. When a photon hits an atom, it can either be scattered or absorbed. In the case of scattering, the photon simply bounces off of the atom.
But when absorption occurs, the photon is actually converted into energy that Excites The Electron Of An Atom To A Higher Energy State . The electron then falls back down to its original state and emits a photon – which is usually of a lower energy (and thus, lower color) than the one that was originally absorbed.
This process of Absorption And Emission Is How Helium Gas Is Able To Absorb Red Light .
So next time you’re looking at a helium-filled balloon, remember that it’s not just reflecting back some of the blue and violet light – it’s also absorbing all of the red!
2. What Colors of Light are Absorbed by Helium Gas? __________________________________
When it comes to light and color, helium is not very picky. This gas will absorb any color of light that hits it. This includes all the colors of the visible spectrum, as well as ultraviolet and infrared light.
In fact, helium is so good at absorbing light that it is often used in optical fiber systems to help keep unwanted light from leaking out.
What Colors of Light are Absorbed by Helium Gas Gizmo
When you think of helium gas, you might think of party balloons or a squeaky voice. But did you know that this gas can also absorb light? In fact, helium is known to absorb certain colors of light more than others.
So what colors does helium gas absorb? Helium is most likely to absorb red and ultraviolet light. This is because the atoms in helium are excited by these wavelengths of light.
When the atoms are excited, they emit energy in the form of heat.
While all colors of light can be absorbed by helium, these two colors are absorbed more readily. So if you’re looking to create a cool effect with your helium-filled balloon, try shining a red or ultraviolet light on it!
Which Photon Energies were Absorbed by Gas a
On November 5, 1895, Wilhelm Conrad Röntgen discovered X-rays while experimenting with electron beams in a gas discharge tube. He found that when the cathode was covered with black paper, a fluorescent screen on the other side of the tube glowed. Roentgen realized that something from the cathode was passing through the paper and making the screen glow.
He dubbed these unheard of rays “X”, for their unknown nature. In 1901 he received the first Nobel Prize in Physics for his discovery.
What Roentgen didn’t know at the time was that he had discovered a new type of electromagnetic radiation, now known as X-rays or gamma rays.
These are high energy photons with short wavelengths that can penetrate human tissue. Medical X-ray machines use this property to take pictures of our insides without having to cut us open!
But not all materials are transparent to X-rays.
Some elements, like lead, are very good at absorbing them. This is why we use lead aprons when getting an X-ray – to protect our bodies from this harmful radiation.
What Happens to the Electron When the Photon is Absorbed?
In short, when a photon is absorbed by an electron, the electron’s energy level increases.
But what happens more specifically? When a photon is absorbed, the electron that absorbs it essentially jumps to a higher energy state.
This can be thought of as the electron moving to a higher orbit around the nucleus of an atom (or molecule).
The amount of energy imparted to the electron depends on the wavelength of the photon – shorter wavelength photons impart more energy than longer wavelength photons. And because electrons can only occupy certain energy levels within an atom, this jump to a higher level may be temporary.
If the extra energy isn’t quickly removed (through heat for example), then it’s possible for the electron to ‘jump’ again to an even higher level.
Bohr Model Gizmo
In the early 1900s, scientists discovered that atoms are composed of smaller particles called electrons. However, they didn’t know exactly how these electrons were arranged inside atoms. In 1913, Danish physicist Niels Bohr proposed a model for the hydrogen atom that explained its emission spectrum.
His model also showed that electrons orbit the nucleus in specific shells.
Today, we use the Bohr model to visualize how electrons are arranged in atoms. The Bohr model is a limited representation of atomic structure, but it’s still useful for introductory chemistry students.
The Bohr model Gizmo™ allows you to explore how the position and energy of an electron depend on its distance from the nucleus. You can also investigate how different elements have different numbers of electrons in their outermost shells.
What is the Energy of the Absorbed Photon
In order to understand the energy of the absorbed photon, we must first understand what a photon is. A photon is a particle of light that has no mass and travels at the speed of light. Photons are emitted when an atom is excited and then returns to its ground state.
The energy of a photon is directly proportional to its frequency; higher frequency photons have more energy than lower frequency photons.
Now that we know what a photon is, let’s talk about how it relates to the energy of an absorbed photon. When a photon is absorbed by an atom, the atom gains energy.
This increase in energy can cause the atom to become excited and emit a photon itself. The amount of energy gained by the atom when it absorbs a photon depends on the frequency of the absorbed photon; higher frequency photons impart more energy than lower frequency photons. In addition, the angle at which the photon strikes the atom also affects how much energy is imparted; photons striking perpendicular tothe atomic surface will impart more energy than those striking at an angle.
So, in summary, when a photon is absorbed by an atom, theatom gainsenergy. The amount ofenergy gainedbytheatomdepends on boththefrequencyof th eabsorbedphotonandtheangleatwhichth ephotostrikes theatomic surface .
Student Exploration: Bohr Model: Introduction
The Bohr model of the atom was developed by Danish physicist Niels Bohr in 1913. It was a revolutionary new way of understanding the structure of atoms and how they interact with light. The model helped to explain many previously unexplained phenomena, such as why atoms absorb and emit certain wavelengths of light.
In the Bohr model, an atom is composed of a small central nucleus surrounded by electrons that orbit in shells around the nucleus. The shells are designated by numbers; the first shell can hold up to two electrons, the second shell can hold up to eight electrons, and so on. Electrons in different shells have different energies; those in higher shells have more energy than those in lower shells.
When an electron jumps from a lower energy shell to a higher energy shell, it emits a photon with an energy equal to the difference between the two levels. For example, if an electron jumps from level 2 to level 4, it will emit a photon with an energy of 2 eV (electron volts). Similarly, when an electron falls from a higher energy shell to a lower one, it absorbs a photon with an equal amount of energy.
This explains why atoms only absorb or emit photons with specific energies; each element has its own characteristic set of absorption and emission lines based on its electronic structure.
The Bohr model is no longer used as a scientific explanation for atomic behavior; it has been superseded by quantum mechanics. However, it remains useful as a conceptual tool for understanding basic ideas about atoms and their interaction with light.
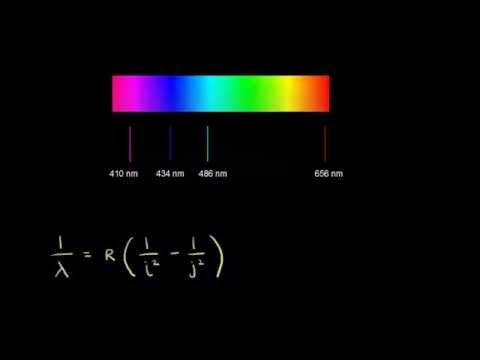
Credit: www.khanacademy.org
Does Hydrogen Absorb White Light?
No, hydrogen does not absorb white light. White light is a combination of all colors of the visible spectrum, and each color is absorbed by different materials. Hydrogen only absorbs certain colors within the visible spectrum, so it would appear transparent to white light.
What Color of Light is Absorbed by Blue?
One of the most interesting things about blue light is that it is both a color and a type of energy. Blue light waves are shorter than other colors, which means they carry more energy. This makes blue light very powerful and useful, but it also means that it can be harmful if we’re exposed to too much of it.
Blue light is absorbed by anything that is blue in color. This includes objects like blueberries, blue clothing, and even the ocean. When these objects absorb blue light, they convert it into other forms of energy like heat or electricity.
What is the Dominant Color of the Hydrogen Spectrum?
The dominant color of the hydrogen spectrum is blue. This is because the hydrogen atom emits light at a wavelength that is in the blue region of the visible spectrum. The blue color is produced when the electron in the hydrogen atom transitions from the n=2 energy level to the n=1 energy level.
When this happens, a photon of light is emitted with a wavelength that corresponds to blue light.
Conclusion
The colors of light that are absorbed by hydrogen gas are those in the ultraviolet and visible portions of the electromagnetic spectrum. These include all the colors except for red. Red light is not absorbed because it is at the lower end of the energy scale, and hydrogen only absorbs high-energy photons.
The reason why we see a blue color when looking at a flame containing hydrogen is because this gas emits light in the blue part of the spectrum when it is excited by heat.
